
Scientists from the University of Oregon in the United States offered the technology electrochemical iron production as an alternative to traditional methods.
The new method, in which an electric current is passed through a liquid rich in materials with a high iron content, does not require high temperatures and can significantly reduce harmful emissions, including greenhouse gases, sulfur dioxide and particulate matter. A team of scientists led by Paul Kempler has demonstrated that particulate iron oxide can be reduced to pure iron in a sodium hydroxide solution at relatively low temperatures of around 80-90°C.
However, natural iron ores are typically high-density, heterogeneous, and full of impurities. Therefore, they are not very suitable for producing iron in this way.
To solve this problem, Kempler and his team, together with researchers Anastasia Konovalova and Andrew Goldman, began to study types of materials similar to iron ore, which could facilitate the scaling up of an environmentally friendly iron production process. First, the researchers prepared iron oxide particles with a large surface area, with internal holes and connecting cavities to study how their shape and size would affect the course of electrochemical reactions at the nanoscale.
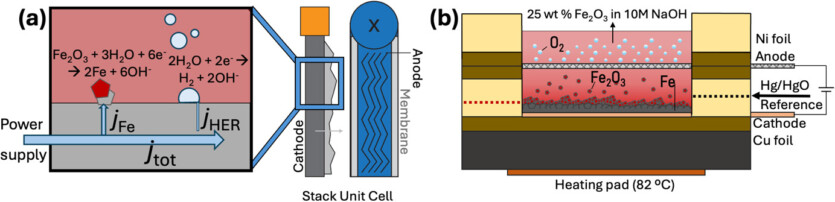
The width of some of these particles was then reduced to micrometers in order to mimic the morphology of natural iron ore. These particles included only a few trace impurities such as carbon and barium. The researchers developed a specialized cathode to extract pure iron from a sodium hydroxide solution that contained iron oxide particles after passing an electric current through the solution.
In the experiments, dense iron oxides were reduced and converted to pure iron at an electric current density of 50 milliamps per square centimeter. The more heterogeneous particles with high porosity and a larger surface area contributed to the more efficient production of pure iron in this way than those created by similarly porous natural iron minerals, hematites.
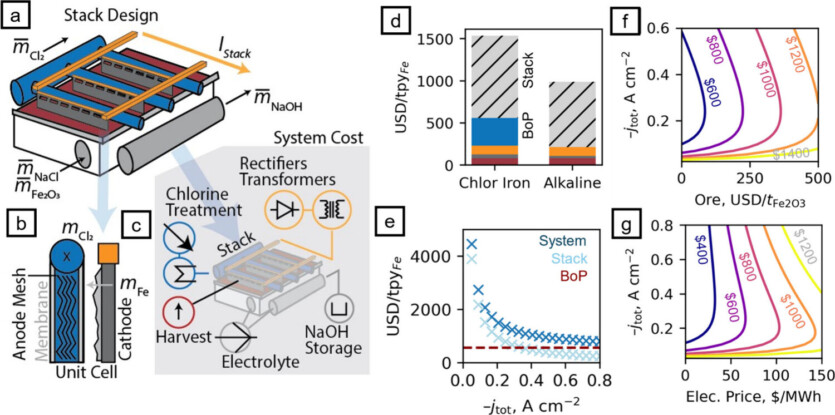
According to the researchers, the potential cost of their electrochemical method of iron production using a current density of 50 milliamps could be less than $600 per metric ton, or 60 cents per kilogram, which is comparable to the cost of traditional iron production methods. The study showed that much higher current densities of up to 600 milliamps per square centimeter, similar to those used in industrial electrolytic cells, can be achieved by using particles with nanoscale porosity. However Further advances in electrochemical cell design and methods are needed to make the iron oxide feedstock more porous before the technology can be commercially applied.
The results of the study were published in the journal ACS Energy Letters
Source: SciTechDaily

Spelling error report
The following text will be sent to our editors: