American physicists from The University of Nevada, Reno conducted an unusual experiment by heating gold with lasers to 18,726°C. For some trillionths of a second the metal remained hard and did not want to melt.
«We were surprised to find that these superheated solids had a much higher temperature than we had initially expected. This was not our initial goal, but this is the essence of science — to discover new phenomena that you did not even know existed», — says the head of the experiment Tom White.
The laws of thermodynamics set a clear upper limit on the amount of heat, that can be absorbed by a solid without a phase transition to a liquid state. Exceeding this limit leads to the so-called «entropic catastrophe» — critical point, at which disordered state atoms of a solid exceeds that of a liquid, which violates the second law of thermodynamics.
This threshold was set at approximately three melting points of the material. Anything higher was considered impossible. In the new study, Tom White and his colleagues used a precisely tuned laser to heat a 50-nanometer-thick gold film. After just 45 femtoseconds, which is less than it takes light to travel the thickness of a human hair, gold atoms began to vibrate furiously.
Using linear accelerator of coherent light at the SLAC National Accelerator Laboratory in California, scientists irradiated overheated gold by pulses of ultra-bright X-rays. The scattered X-ray photons demonstrated the speed of atomic vibrations. This allowed the researchers to directly measure the temperature of the ions.
In 1988, physicists Hans Fecht and William Johnson introduced the concept of «entropic catastrophe», arguing that no solid can have a higher entropy than its own liquid counterpart. However, these limits are only valid under certain conditions, when materials are heated slowly enough to maintain thermal equilibrium.
A new experiment Tom White and his team were able to circumvent these limitations. The researchers heated gold faster than its atoms could rearrange themselves.
«It is important to clarify that we have not violated the second law of thermodynamics. We have demonstrated that these catastrophes can be avoided by heating materials very quickly — in our case, in trillionths of a second», — explains Tom White.
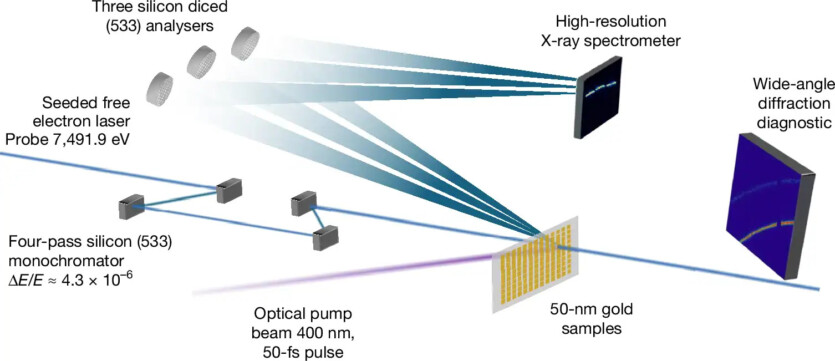
The success of the experiment was ensured by the use of inelastic scattering of X-rays — in a manner similar to the measurement of atomic radar velocity. When X-rays collide with vibrating atoms, their frequency shifts slightly due to the Doppler effect. The wider the frequency spread of scattered photons, the hotter the atoms.
«We have good methods for measuring the density and pressure of these systems, but not the temperature. In these studies, the temperatures are always estimated with huge errors, which really supports our theoretical models», — emphasized is a researcher at SLAC and one of the study’s leaders Bob Nagler.
Using a few hundred scattered X-ray photons, the researchers obtained reliable temperature readings directly, without the need for modeling. Understanding how materials behave under extreme temperatures and pressures is key to designing fusion reactors, modeling the interior of other planets, and other pressing scientific questions.
Researchers studying the «dense warm matter» — exotic state observed in the interior of giant planets and in the first moments of fusion reactions have long worked with the uncertainty associated with measuring thermal characteristics. This study proposes a new direct method for calibrating such models.
For research in the field of inertial fusion, it is necessary to know how hot fusion fuel targets become during fusion. The new method allows for such measurements. Researchers believe, based on preliminary data, that silver may also surpass its entropy limit.
Normally, in traditional heating and melting, heat drives atoms out of equilibrium and disrupts the ordered crystal lattice. However, in this experiment, there was simply no time for that. The crystal lattice of gold did not have time to expand. The researchers claim that the lack of expansion prevented the onset of an entropic catastrophe. Without expansion, the entropy of a solid will never lead to a phase transition.
«The intersection of the two entropy curves is effectively eliminated by ultra-fast intense heating. Overheating may not have an upper limit of», — the researchers say.
The challenge now is to understand how widespread this phenomenon is. Can other metals behave in the same way? Can this be used to create materials with new properties?
The results of the study are published in the journal Nature
Source: ZME Science

Spelling error report
The following text will be sent to our editors: