
American chemists from Northwestern University and Purdue University have created a nickel-based catalyst for recycling of mixed plastic waste without the need for preliminary sorting.
Household goods and food packaging are predominantly made of plastic. Almost all of this plastic consists of polyolefins — polyethylene and polypropylene, key components of disposable waste. Less than 10% of them are recycled.
This is due to the fact, that carbon-carbon bonds in polyolefins are very durable and waste decomposes very slowly. Plastic waste remains in landfills and in the environment for decades, decomposing into hazardous microparticles.
Plastic recycling is also a challenge. Yogurt cups cannot be recycled with milk cartons. Even a tiny piece of unsuitable polymer can spoil the entire batch. Sorting plastic waste, whether automated or manual, is a very complex and slow process. As a result, plastic recycling rates remain extremely low. However, the catalyst, created by American chemists, works even with dirty plastic waste, containing polyvinyl chloride.
Over 220 million tons produced annually polyolefins. According to a 2023 Nature report, the global recycling rate for this waste is less than 10%, and in some cases, less than 1%. Sorting is so difficult, that most mixed batches of plastic waste immediately sent to a landfill.
“One of the biggest obstacles in plastic recycling has always been the need to carefully sort plastic waste by type. Our new catalyst can bypass this costly and time-consuming step in the processing of conventional polyolefins, making recycling more efficient, practical and cost-effective than existing strategies”, — says the study’s senior author Tobin Marks.
Recycling system, proposed by american scientists, based on a single-centered organonickel catalyst, fixed to a “super acid” carrier made of sulfated aluminum oxide. According to the results of tests in the laboratory, this catalyst converted low-value polyolefins into liquid oils and waxes, that could be reused as a lubricant, fuel, or even as candle wax.
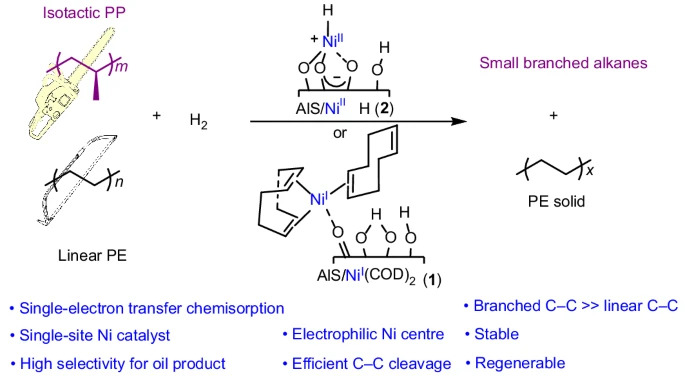
In contrast to heat treatment, in particular, pyrolysis, which involves sintering plastic at temperatures of 400-700°C until it will crack and not turn into a disordered mass, the nickel-based catalyst operates at temperatures around 200°C. It uses hydrogen to gently break carbon bonds.
Compared to traditional nickel nanoparticles, this catalyst proved to be much more effective. In one test, it converted almost all of the isotactic polypropylene for liquid hydrocarbons within 20 minutes. The conversion rate of iPP was 16 grams per gram of catalyst per hour.
At the same time, the catalyst distinguished between different types of plastic among the total pile and selectively recycled polypropylene, practically without touching polyethylene. This is the first time, that such a selective sorting of plastic waste has been achieved by chemical means.
Polyvinyl chloride is very problematic to recycle. When heated, it releases hydrochloric acid, which destroys equipment and damages catalysts. However, after the researchers added PVC to the mixed plastic waste, the catalyst began to work even better.
“Adding PVC to the mix during the recycling process has always been prohibited. But it seems to make the process even better. It’s crazy. Nobody expected this”, — said one of the study’s leaders, a researcher from Northwestern University Josi Kratish.
The data obtained by the scientists confirmed, that a small amount of hydrochloric acid, released by PVC, is able to restore the acid carrier, helping the catalyst to break carbon bonds. Among the problems encountered, are the catalyst’s vulnerability to air and a decrease in efficiency after each use. However, it can be restored with an aluminum-based treatment.
The results of the study are published in the journal Nature Chemistry
Source: ZME Science

Spelling error report
The following text will be sent to our editors: