
An international team of researchers, led by scientists from The US Department of Energy’s SLAC National Accelerator Laboratory, has succeeded in creating a solid binary gold hydride for the first time.
This compound consists of exclusively from gold atoms and hydrogen. Initially, the researchers sought to study, how hydrocarbons — molecules composed of carbon and hydrogen, turn into diamonds under extreme pressure and high temperature.
As part of the experiments, conducted by at the european Free-Electron X-ray Laser (XFEL) in Germany, hydrocarbon samples were placed on a thin layer of gold foil, designed exclusively for absorption X-rays and heat transfer to relatively weakly absorbing hydrocarbons. However, along with the formation of diamonds, the researchers unexpectedly observed the formation of gold hydride.
“This was unexpected because gold is usually chemically very boring and inert, so we use it as an X-ray absorber in these experiments. These results suggest, that under extreme conditions, where the effects of temperature and pressure begin to compete with traditional chemical processes, many new chemical reactions can potentially be discovered and these exotic compounds can be formed”, — says lead author of the study, a researcher at SLAC Mango Frost.
To obtain the relevant results, the scientists compressed the hydrocarbon samples to a degree, that exceeds the pressure in the Earth’s mantle, using a chamber with diamond anvils. After that, they exposed the samples of X-ray pulses from the European X-ray laser XFEL, heating them to a temperature of 1.9 thousand °C. They analyzed the scattering of X-rays from samples, tracking structural changes.
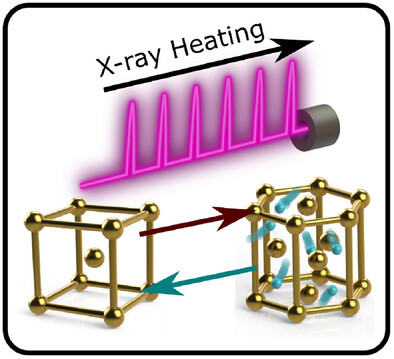
The results confirmed, that the carbon atoms were lined up in a diamond lattice. However, at the same time hydrogen atoms reacted with the gold foil to form gold hydride. Under the conditions, created in the experiments, hydrogen was in a dense “superionic” state, in which hydrogen atoms moved freely inside a rigid golden lattice. This behavior increased the conductivity of the gold hydride, opening up new opportunities to understand the behavior of materials at extreme pressures and temperatures.
Since hydrogen scatters X-rays poorly, it is extremely difficult to study it in this way. However, in this case, superionic hydrogen interacted with much heavier gold atoms, and scientists were able to observe the effect of hydrogen on the scattering of X-rays by the gold lattice.
Gold hydride makes it possible to study dense atomic hydrogen under conditions, that can be applied to other situations, that are not available for experimental research. For example, dense hydrogen is part of the interior of some planets. Its study in the laboratory can give us more information about these distant worlds. It can also provide new insights into the processes of fusion inside stars, like our Sun, and help develop technologies for using fusion energy on Earth.
This study also reveals new opportunities for studying chemical processes. It was discovered, that gold, which is normally considered inert, forms a stable hydride under extremely high pressure and temperature. In fact, it is probably only stable under these extreme conditions, as gold and hydrogen separate upon cooling.
The results of the study were published in the journal Angewandte Chemie International Edition
Source: SciTechDaily

Spelling error report
The following text will be sent to our editors: