
Researchers from the Massachusetts Institute of Technology (MIT) in the United States have developed a sodium-air fuel cell, which can power heavy electric vehicles, including airplanes.
Currently, in the era of transition from fossil fuels to alternative, electric batteries are becoming one of the key elements. Li-ion batteries are still the most versatile solution for electric vehicles. However, they also have their own capacity limitations, which creates difficulties in adapting them to the requirements of heavy electric vehicles, such as airplanes, ships, and trains.
Metal-air batteries have been proposed as an alternative, but are still under development. Meanwhile, researchers at MIT have developed a completely different approach to using this concept.
MIT engineers managed to achieve higher energy density by replacing the liquid electrolyte with a solid fuel cell. In their experiment, the scientists used two vertical glass tubes that were connected in the middle with a solid ceramic electrolyte and a porous air electrode. One of the tubes contained a liquid sodium, and in the other — air. The chemical reaction produced sodium oxide and energy. By controlling the humidity of the air flow, the scientists successfully generated 1700 W⋅h per kilogram of each battery. An electric airplane, for example, requires an energy density of 1000 Wh per kg.
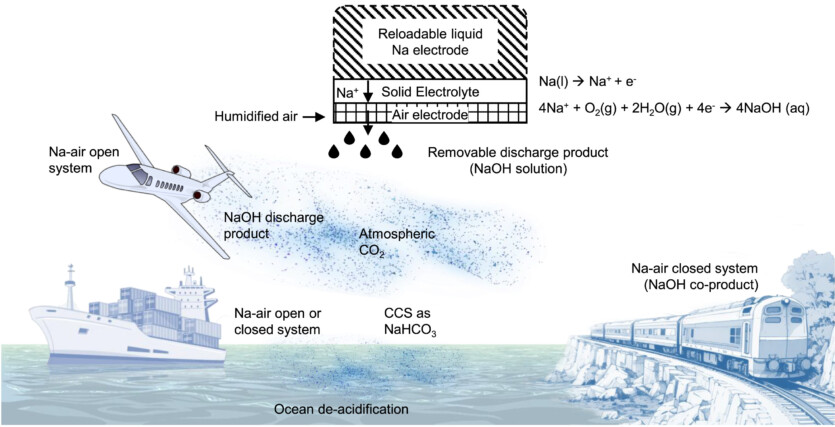
Unlike batteries, where components need to be recharged, fuel cell components are used and easily replaced, which can be done much faster than charging battery components. In addition to this, the fuel component is free from the risk of thermal overheating.
The sodium oxide released by the fuel cell reacts with moist air to form sodium hydroxide. The latter reacts with carbon dioxide to form sodium carbonate, and then — sodium bicarbonate, or baking soda.
In addition to cleaning the atmosphere from CO2 sodium bicarbonate can enter the oceans and reduce their acidification level. These solutions have been proposed to combat climate change but have not been implemented due to high costs.
Unlike lithium used in modern batteries, which is available in certain regions and requires careful processing, sodium can be obtained from salt anywhere in the world. The researchers plan to create brick-sized fuel cells to power large drones to demonstrate their concept, and then develop larger ones for airplanes and ships.
Graphene helped: engineers extend operation of hydrogen engines to 200,000+ hours
The results of the study are published in the journal Joule
Source: Interesting Engineering

Spelling error report
The following text will be sent to our editors: