
Chinese researchers have developed a new graphene-coated catalyst with ultra-low platinum content that provides highly efficient production «green» hydrogen on an industrial scale.
In large-scale production «green» hydrogen the key role is played by the process of water electrolysis using a proton exchange membrane. The most common material used in conjunction with carbon is platinum, which acts as a cathode catalyst. Platinum effectively binds hydrogen and is highly resistant to acidic environments. However, the use of large amounts of platinum makes hydrogen production in this way very expensive and limits its widespread use.
A group of Chinese researchers, led by Prof. Dehui Deng and Prof. Liang Yu from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), worked with Prof. Junlin Lu from the University of Science and Technology of China and Prof. Hongmei Yu from DICP to develop a catalyst with high efficiency and stability for use in acid hydrogen production. The scientists managed to create a unique «chainmail» catalyst consisting of a cobalt and nickel nanalloy placed in a single layer of graphene.
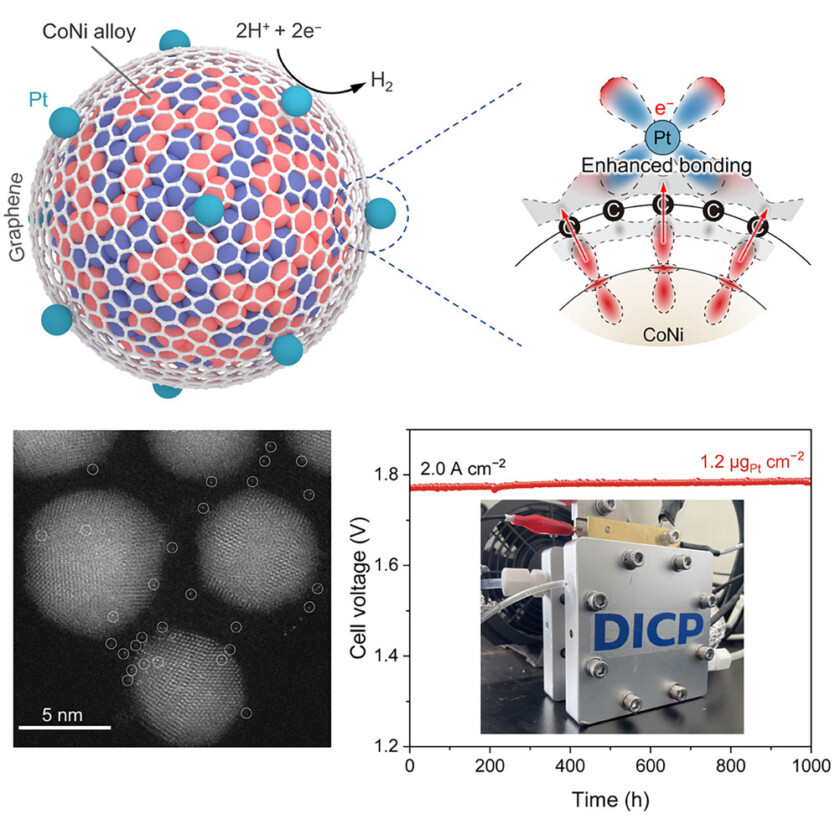
Scientists have found that the transfer of electrons from an alloy cobalt and nickel into the surrounding carbon layer. This process, in combination with the 3d-2p electronic interaction, leads to the accumulation of an uneven distribution of π-electronic states on the graphene surface, which plays a crucial role in improving catalytic properties.
After the deposition of individual platinum atoms using the atomic layer deposition, enriched with asymmetric π-electrons demonstrated a unique effect of confining platinum atoms. The transfer of electrons from the nickel-cobalt alloy to platinum through the graphene layer led to the formation of an electron-rich platinum center, which optimized hydrogen adsorption energy and promoted its desorption, thereby increasing catalytic activity. In addition, the strong interactions between the asymmetric π-electrons and the 5d orbital of platinum enhanced the structural stability of the platinum centers, increasing the durability of the catalyst.
Using this catalyst, the researchers assembled an aqueous electrolyzer with a proton exchange membrane-electrode that achieved ultra-high current density 4.0 A·cm-2 at 2.02 V and retained excellent durability of more than 1 thousand hours at 2 A·h-2using only 1.2 µg of platinum per cm2. They also assembled a 2.85 kW PEM water electrolyzer using this catalyst, which operated stably for more than 300 hours at an industrial current density of 1.5 A·cm-2.
The results of the study were published in the journal Joule
Source: SciTechDaily

Spelling error report
The following text will be sent to our editors: